Research news
Enantioselective 1,2-Anionotropic Rearrangement of Acylsilane through a Bisguanidinium Silicate Ion Pair
Researchers from NTU (Prof. Choon-Hong Tan) and SUTD (Dr. Richmond Lee) have published a collaborative work on the high impact Journal of American Chemical Society. The work centers on asymmetric acylsilane rearrangement by a bisguanidine ion-pair catalyst. Experiments were first carried out by the NTU lab and computational studies followed in SUTD. Importantly, this paper offers a major step forward in asymmetric silicon chemistry and understanding of its reaction mechanism.
For the full article, please refer to https://pubs.acs.org/doi/10.1021/jacs.7b13056
Half-Sandwich Ruthenium Phenolate–Oxazoline Complexes: Experimental and Theoretical Studies in Catalytic Transfer Hydrogenation of Nitroarene
Combined experimental and theoretical studies on a half-sandwich ruthenium catalyst’s reactivity were carried out by our cluster’s faculty fellow Dr. Richmond Lee and his long-standing collaborator Prof. Weiguo Jia from the Anhui Normal University, China. This paper examines the role of the catalyst class towards transfer hydrogenation of nitroarenes to anilines which is very relevant to chemical industries. Mechanistic insights gained with theoretical studies could further strengthen the design of more efficient catalysts which is the ultimate aim of Richmond’s research goals here at SUTD. This is his first work as a corresponding author in SUTD published in a major peer-reviewed journal in the field of organometallics.
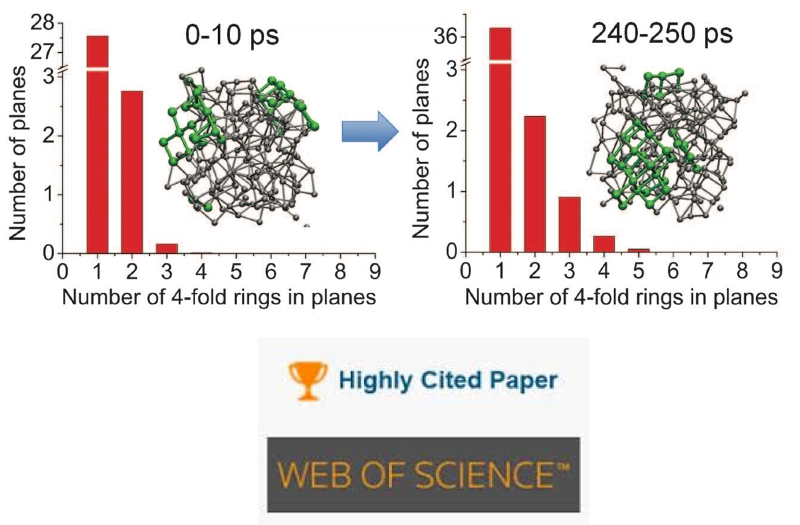
Breaking the Speed Limits of Phase-Change Memory
The Loke group is very humbled to receive a Web of Science Index’s “Highly Cited Paper” honor for our lab’s effort to make Big Data more accessible for everyone.

Rationally Design Bright Near-Infrared Fluorophores
Fluorophores with near-infrared (NIR) emissions play a crucial role in numerous bioimaging and biosensing applications. These NIR fluorophores afford highly attractive optical properties, such as deep penetration depths, good signal-to-noise ratios, and minimal tissue damages. Recently, Assistant Professor Liu Xiaogang and his co-workers have rationally developed a new class of near-infrared fluorophores with bright one-photon and two-photon emissions. In this work, Dr. Liu Xiaogang and their co-workers presented a rational molecular design strategy, which is expected to inspire the molecular engineering of other high-performance near infrared fluorophores as well.
Their paper will appear in “Chemistry – an European Journal“. It has been selected as a “Hot Paper” by the Editor. “Hot Papers are chosen by the Editors for their importance in a rapidly evolving field of high current interest”.
Assistant Professor Liu Xiaogang joined SUTD and started the Fluorescence Research Group in Apr 2017.