SUTD researchers turn to age-old molding technique to 3D-print cell-laden vascular models
SUTD - Terry Ching, Jyothsna Vasudevan, Shu-Yung Chang, Hsih Yin Tan, Anupama Sargur Ranganath, Chwee Teck Lim, Javier G. Fernandez, Jun Jie Ng, Yi-Chin Toh and Michinao Hashimoto
The biofabricated vascular models will pave the way for drug development and deeper understanding of cardiovascular diseases.
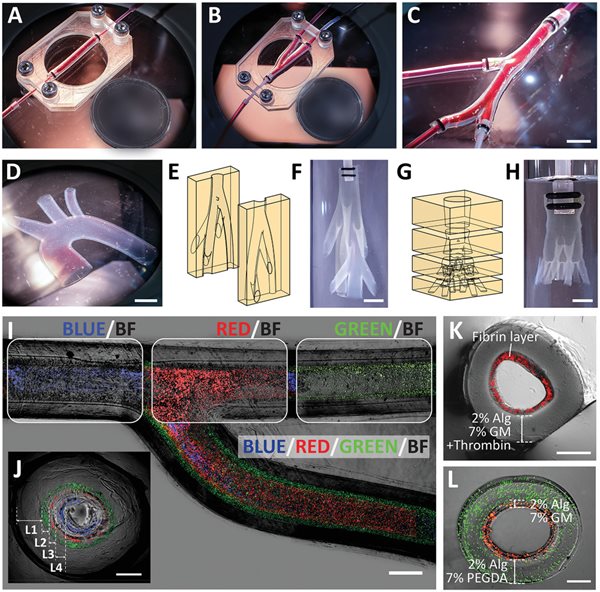 Photographs of planar vascular models: A) straight vascular construct, B) bifurcating vascular construct, C) vascular constructs with multiple branches, and D) aortic arch. Illustrations and photographs of 3D vascular models: E,F) a nonplanar biomimetic branching network (one inlet to seven branches), G,H) a nonplanar vascular tree (one inlet to nine branches). Image of a bifurcating vascular construct consisting of four layers (as depicted by the encapsulated fluorescent beads of different colors) shown in I) top view and J) cross-sectional view. Cross-sectional image of multilayered vascular construct consisting of K) Alg-GM in the outer layer and fibrin hydrogel in the inner layer, and L) Alg-PEGDA in the outer layer and Alg-GM in the inner layer. Scale bars: (I) 1 mm, (C,D,F,H) 5 mm, (J–L) 500 µm. Circular reference (diameter): A, B) 18.50 mm.
Photographs of planar vascular models: A) straight vascular construct, B) bifurcating vascular construct, C) vascular constructs with multiple branches, and D) aortic arch. Illustrations and photographs of 3D vascular models: E,F) a nonplanar biomimetic branching network (one inlet to seven branches), G,H) a nonplanar vascular tree (one inlet to nine branches). Image of a bifurcating vascular construct consisting of four layers (as depicted by the encapsulated fluorescent beads of different colors) shown in I) top view and J) cross-sectional view. Cross-sectional image of multilayered vascular construct consisting of K) Alg-GM in the outer layer and fibrin hydrogel in the inner layer, and L) Alg-PEGDA in the outer layer and Alg-GM in the inner layer. Scale bars: (I) 1 mm, (C,D,F,H) 5 mm, (J–L) 500 µm. Circular reference (diameter): A, B) 18.50 mm.
Cardiovascular diseases (CVDs) remain to be one of the leading causes of mortality around the world. There is an urgent need for improved vascular models, anatomically and biologically, to advance our understanding of disease progression. Such understanding can lead to the development of new therapeutic interventions.
While scientists conventionally rely heavily on animal models to help shed light on the pathophysiology of CVDs and drug development, they are limited by their ability to predict toxicity in humans. “Given the differences in the underlying molecular, cellular and physiological mechanisms between animals and humans, animal models do not necessarily provide us with an accurate understanding of human physiology,” explained Associate Professor Yi-Chin Toh, corresponding author from Queensland University of Technology (QUT).
To create alternative models more relevant to humans, scientists turn to engineering tissues using living human cells. “To identify key cellular and molecular contributors to human physiology and pathophysiology, living human cells are utilised in the field of tissue engineering to build artificial models with defined composition at the cell, tissue and organ level,” explained Professor Toh. By directly using human cells, these engineered tissues could provide insights into normal human organ function and disease pathophysiology, as well as more accurately predict the safety and efficacy of investigational therapeutics in humans.
One of the technologies currently employed in the field of tissue engineering is 3D bioprinting – where living human cells are mixed with bioinks and deposited in a specific manner to recapitulate the microenvironment of native organs. However, fabricating the intricacies found in organs using current bioprinting methods is still challenging due to technology limitations. One of the difficulties is in recapitulating the complexities of blood vessels. For example, blood vessels branch hierarchically, where larger vessels branch into multiple smaller vessels across various length-scale. Blood vessels are also multilayered, each layer consisting of different cell types.
“The printed bioink must serve a dual function of supporting living cells and providing structural integrity to the vascular construct. Existing bioink suitable for living cells are typically soft and fragile, making it challenging to 3D print the complex architecture of blood vessels directly,” said principal investigator, Associate Professor Michinao Hashimoto from the Singapore University of Technology and Design (SUTD). “Because of the existing limitations of 3D bioprinting, we decided to deviate from relying entirely on 3D printing to fabricate the vascular constructs.”
The team developed a fabrication technique inspired by the age-old molding method. 3D printing was used to fabricate the molds for the vascular networks. However, unlike conventional molding processes where the filled liquid material solidifies en masse, the team introduced a unique solidifying approach.
The technique involved using a two-part, 3D-printed mold consisting of hydrogels of poly(ethylene glycol) diacrylate (PEGDA). PEGDA hydrogel was selected because it could behave like a sponge to soak up calcium ions, which are responsible for crosslinking the selected bioink. When the alginate-containing bioink was perfused through the mold cavity, the calcium ions within the mold diffused radially into the mold cavity. The diffusion of the calcium ions prompted rapid ionic crosslinking of the alginate present in the bioink, forming a tubular construct.
“We can effectively control the thickness of the vessel wall by varying the duration the bioink is held in the mold cavity. Subsequently, we can perfuse a buffer solution through the mold to remove the uncrosslinked bioink,” said lead author Terry Ching, PhD student from SUTD.
Using this technique, the researchers successfully engineered freestanding, branching, multilayered and perfusable vascular networks. “Importantly, we were able to mix other bioactive materials into the bioink to make the microenvironment more suitable for human vascular cells,” added Ching. The team incorporated relevant vascular cells in a configuration similar to that found in vivo. The team also mounted their freestanding vascular constructs on an expandable balloon to simulate the cyclical loading that coronary arteries experience in vivo.
This fabrication technique’s versatility and multifaceted possibilities should give users more control in accommodating a variety of bioinks and patient-specific cells. “Ultimately, we hope to use these biomimetic vascular constructs to benefit future research in the mechanistic understanding of CVDs as well as models to evaluate therapeutic interventions,” explained Associate Professor Michinao Hashimoto.
The paper “Biomimetic Vasculatures by 3D-Printed Porous Molds” was published in the journal, Small. This work is a joint effort between Associate Professor Michinao Hashimoto from SUTD’s Soft Fluidics Lab and Professor Yi-Chin Toh from QUT’s MicroTE lab. This work was highlighted on the cover of Small, volume 18, issue 39.
Acknowledgements:
T.C. thanks Ministry of Education (MOE), Singapore, for awarding the President's Graduate Fellowship. J.J.N. acknowledges Abbott for providing the drug-eluting stents and balloons. M.H. acknowledges Agency for Science, Technology and Research (A*STAR) (A*STAR-AMED joint grant, A19B9b0067) and MOE, Singapore (Academic Research Fund (AcRF) Tier 2, MOE2019-T2-2-192) for the project funding. Y.C.T. acknowledges MOE, Singapore (Tier 1, R-397-000-298-114), Australian Research Council (ARC) Future Fellowship (FT180100157), and Discovery Project (DP200101658).
Reference:
Biomimetic Vasculatures by 3D-Printed Porous Molds, Small.(DOI: 10.1002/smll.202203426)